Biology & Chemistry Research
Additional Navigation

Prepare for Your Career in Biology or Chemistry Through Hands-On Research
Learn to succeed in your field of study by conducting research with hands-on experiments in nearly every science class you take. In Liberty University’s Department of Biology & Chemistry, you will participate in labs taught by credentialed and experienced faculty.
Also, in our 400-level courses, you will have the opportunity to conduct your own research projects or do research simulations. Additionally, you could participate with faculty on their ongoing research projects.
Experience like this has given students the opportunity to present their research results at both regional and national scientific meetings, including the Virginia Academy of Science. Research experience can also help you gain internship opportunities, attend graduate school, and find successful employment.
LU Undergrads Gain Lab Research Experience from Liberty News on Vimeo.
Read about Bio/Chem research in “Overachieving Undergraduate Programs” in the Liberty Journal.
Biology and Chemistry Faculty Research Opportunities Include:
The Adebola Lab – Aquatic Ecosystems
Dr. Tunde Adebola
View Dr. Adebola’s biography
Dr. Adebola is a fish ecologist with graduate degrees in marine and environmental science, and public policy. Through his research work, he seeks to understand anthropogenic impacts in aquatic ecosystems. His interests range from learning how humans impact aquatic food webs through activities like fishing, agriculture, development, etc., to seeking how environmental degradation can contribute to pathologies in human populations. Dr. Adebola uses a variety of tools including ecological models and geospatial statistics with the aim to inform environmental management and move society toward sustainable practices that promote better environmental stewardship.
The Allen Lab – Algae Biofuels
Dr. Todd Allen
View Dr. Allen’s biography
Algae can be grown to have a high protein and/or lipid content, which can be used for animal feed, biofuel, or both. Dr. Allen’s group uses GC/MS (Gas Chromatography coupled with Mass Spectrometric Detection) for quantifying the total lipid content in samples obtained from algae biofuels companies who are developing cost-effective ways to optimize growth and harvesting techniques to convert the lipid fraction of the algae biomass into various types of biofuels.
This work provides students with hands-on, real-world, interdisciplinary training in both chemistry and biology. It will provide them with valuable, transferable skills and knowledge, and prepare them for employment, graduate research, or medical school.
Examples:
- Students will apply fundamental principles of stoichiometry, kinetics, and equilibrium learned in their chemistry and biology courses to the preparation and analysis of samples.
- Students will acquire valuable chemistry and biology research experience through literature searches, in sample/standard preparation techniques, in the use of state-of-the-art instrumentation, as well as in data analysis, interpretation, and reporting.
- Students will learn how to effectively summarize and communicate scientific information.
The Becker Lab – Amphibians & Their Microbial Symbionts
Dr. Matthew Becker
View Dr. Becker’s biography
The Becker lab is currently conducting two studies to understand the role of symbiotic microbes in the health and conservation of local amphibian species.
The impact of emerging infectious diseases on Virginia salamander populations.
In recent decades, amphibians have experienced unprecedented population declines, leading to many species extinctions worldwide. A large number of these declines and extinctions are occurring in protected areas, such as national parks, and are due to two amphibian diseases: chytridiomycosis and ranaviral disease. The impact of these two diseases in the southeastern United States is unknown and is hard to measure without long-term studies of infected populations.
Therefore, a main goal of the Becker lab is to set up long-term monitoring sites of local salamander populations and measure the impact of disease on population dynamics over time. Study species for this project include the eastern newt (Notophthalmus viridescens) and the Peaks of Otter salamander (Plethodon hubrichti), an endemic species with a very limited distribution along the Blue Ridge Mountains.
The microbiome of the Peaks of Otter Salamander
Animals are host to a diverse community of symbiotic microorganisms. We are currently discovering the significance of microbes to the health and normal function of the hosts they inhabit. In particular, some amphibian species host a wide array of bacteria on their skin that secrete compounds to prevent colonization of infectious pathogens such as the fungus Batrachochytrium dendrobatidis, which is responsible for the disease chytridiomycosis.
Recent studies have shown that some of these beneficial bacteria can be used as probiotics to prevent chytridiomycosis in highly susceptible amphibian species. In an effort to understand the ecology of amphibian cutaneous microbial communities and further conservation strategies with the use of probiotics, the Becker lab uses microbiological and molecular techniques to investigate how these microbes interact with each other, their host, and B. dendrobatidis. Study species for this project will include several local salamander species, including the Peaks of Otter salamander (Plethodon hubrichti).
The Brophy Lab – Vertebrate Ecology & Systematics
Dr. Timothy Brophy
View Dr. Brophy’s biography
Dr. Brophy’s graduate research focused on ecology and systematics of salamanders and turtles, but he has broad interests in all vertebrates. Dr. Brophy has recently worked on several research projects including ecological studies of the Peaks of Otter Salamander and baraminological analyses of landfowl, waterbirds, fossil horses, salamanders, and cucurbits. His team is currently working on a lungless salamander baraminology project that includes hybridization, taxonomic, morphological, molecular, fossil, and biblical datasets.
The Fulp Lab – Modulation of Cannabinoid Receptors
Dr. Alan Fulp
View Dr. Fulp’s biography
The endocannabinoid system is made up of receptors, transporters, endocannabinoids, and enzymes that are involved in the synthesis and degradation of endocannabinoids. Modulation of the endocannabinoid system has the potential to treat a variety of disorders. One area of research that Dr. Fulp is currently pursuing is the development of peripherally selective compounds, compounds that do not penetrate the CNS, that modulate cannabinoid receptors or endocannabinoid levels. These projects involve using synthetic organic chemistry to generate novel compounds with the desired pharmacological activity. Another area of research that Dr. Fulp is currently pursuing is the development of novel synthetic methodology. These projects focus on developing tandem reaction to make synthetically useful intermediates.
The Gillen Lab – Local Water Quality Research
Dr. Alan Gillen
View Dr. Gillen’s biography
Dr. Gillen and his lab students are interested in assessing local water quality. He and his students are tracking the incidence of water parasites and pathogens in local streams and lakes. His major research interests are the detection of natural coliforms, fecal coliforms, water parasites and pathogens. He is using traditional water quality test methods, Giardia rapid tests (ELISA antigen SNAP tests), and Coliscan Membrane Filtration tests. Samples are taken from local ponds, lakes, and streams to assess water quality in the Spring of 2025 compared with those taken in previous years (2016-2024). The results of this research educate students regarding good public health, water quality, and the importance of controlling pathogenic and parasitic diseases in local waters.
The Gilley-Connor Lab – Animal Husbandry
Dr. Kayla Gilley-Connor
View Dr. Gilley-Connor’s biography
Animal Husbandry
Whether you are wanting to go into vet school, grad school, research, or go straight into zoo keeping, experience in animal husbandry helps build skills and experience that is marketable post-graduation.
Depending on the needs from the lab, your level of interest, and how long you’ve been helping care for animals, you will be asked to do a variety of tasks with rodents, including:
- Handling
- Body condition checks
- Feeding, watering, and cage changes
- Insuring animals have adequate enrichment available
- Sub-cutaneous administration of fluids (for those with serious interest and have shown dependability)
- Colony organization
- Breeding management (pairing and weaning of pups)
Liberty has amazing zoo and organismal biology programs, so we love to be able to offer these types of positions for students in those majors to make them competitive when looking for jobs. See below for qualifications:
- Available for students as early as second semester Freshman
- Looking for workers who are willing to check in on animals on the weekend if necessary
- Willingness to learn, communicate, and are dependable
- Especially as an animal care worker, the quality of your work and dependability directly influences our animals’ well-being. As researchers, we are trusting you to make sure we have happy and healthy animals
- No background experience necessary!
The Gilley-Connor Lab 2 –Diabetes, Depression, & Dementia
Dr. Kayla Gilley-Connor
View Dr. Gilley-Connor’s biography
Diabetes, Depression, & Dementia
We will start with exploring how type 1 diabetes and depression are related in male and female preclinical subjects.
Moving forward:
Once our diabetic model is established, we will take our model forward and explore these outcomes:
- Increased depression and anxiety in diabetic males and females
- Increased risk for dementia in diabetes
- The protective role of estrogen and how menopause influences depression, anxiety, and cognition in diabetes.
Interested in Neuro Research?
If you are interested in research experience, you will need to apply for consideration. We have limited spots, but we are just looking for enthusiastic students committed learning new things and wanting to being a team player! No prior experience necessary!
Before you apply:
While many students have preference for bench work skills including tissue processing, microscopy, etc., there are many day-to-day activities that are less than glamorous. We certainly want to offer you the opportunity to build the skills you want, however, if you are not interested in helping maintain a clean space, weighing animals, timing behavior, data processing, etc., then I would suggest exploring opportunities in a different lab.
Building Skill Sets
Research opportunities in the BIOL 495 course will focus on building several skill sets. The research approach is multidisciplinary, including areas of neuroscience, histology and cell biology, psychology/behavioral science, basic animal care and health monitoring, and components of immunology. With that in mind, the skill outcomes reflect this interdisciplinary nature. No matter the specific project, in this research lab students should expect to build skills in:
– Animal husbandry (cage cleaning and animal care such as body weights and blood glucose levels)
– Behavioral evaluation in areas of affect and cognition
– Brain tissue collection
– Brain tissue processing (cryosectioning, immunofluorescent staining, golgi staining)
– Microscopy and histological quantification (counting central immune or neurogenic cells, evaluating neuronal arborization, etc.)
– Data management
– Science communication (presenting data at lab meetings and presenting a poster at research week)
The Goff Lab – Conservation Physiology and Ecology of Amphibians
Dr. Cory Goff
View Dr. Goff’s biography
Research in the Goff lab focuses on Conservation Physiology, the use of physiological metrics (i.e. body condition and stress response) to assess the impacts and responses to environmental conditions and their effects on population health. Current research centers around the ecology and conservation of the Peaks of Otter Salamander (Plethodon hubrichti), a montane endemic to central Virginia. We are conducting long-term assessment of salamander densities, population metrics, and range limits to understand their ecology, as well as new research on population health. We started a multi-year comparative study on the salamander from different elevations, as well as allopatric and sympatric sites (where it is in competition with another similar species) within its range. We perform non-invasive hormone collection to quantify corticosterone release rates, the primary glucocorticoid hormone involved in the stress response. Analysis of corticosterone release rates in combination with body condition indices can be used as metrics to determine the population health and effects of both abiotic factors and competition.
The Harris Lab – Aquatic Ecology
Dr. Kyle Harris
View Dr. Harris’ biography
Dr. Harris conducts research in the area of aquatic ecology. Current undergraduate projects focus on crayfish ecology in relation to ectosymbionts, ecotoxicology, and predator/prey dynamics. These projects are a collaborative effort with faculty in the areas of analytic chemistry, histology, ecology, and microbiology. Students present their findings annually at the Virginia Academy of Science and have opportunities to present at other regional and national research meetings (e.g. Association of Southeastern Biologists and Ecological Society of America).
Lab and field-based projects on crayfish ecology include:
- The microbial assembly on crayfish along stream continuums in relation to crayfish ectosymbionts. This project utilizes molecular research (DNA extractions, PCR, QIIME2 sequencing analysis) to further unravel the role of obligate crayfish ectosymbionts (Branchiobdellidans) on community assembly. Students apply techniques learned in microbiology, genetics, and environmental biology.
- The natural history of crayfish ectosymbionts (Branchiobdellidans) in Central Virginia. This project is cataloging Branchiobdellidans and other crayfish epifauna in Central Virginia streams from associated project field sampling. Students apply skills with taxonomic keys introduced in organismal courses.
- The effects of pollutants on crayfish and crayfish ectosymbionts. Freshwater ecosystems are impacted by a wide variety of pollutants. This project investigates how sub-lethal levels of pollutants (e.g. herbicides) affect non-target organisms in streams. Students apply techniques learned in analytic chemistry, histology, and environmental science.
- The impact of microplastics on rural and urban streams. This project investigates the presence and abundance of microplastics in stream environments with special attention given to crayfish and associated biota (e.g. fish and queen snakes). Students apply techniques learned in ecology, environmental biology, and analytic chemistry.
- The effects of crayfish (predators) on the development of amphibians (prey).Interactions between predators and prey can result in morphological and behavioral plasticity of prey. This project investigates how the presence of crayfish influences the development of amphibians.
The Hedrick Lab – Human Disturbance on Aquatic Ecosystems
Dr. Lara Hedrick
View Dr. Hedrick’s biography
Dr. Hedrick is a new faculty member, and she is researching the impacts of human disturbance on aquatic ecosystems. Dr. Hedricks’s research will focus on the new ecosystem created by the expansion of Lake Hydaway. Preliminary research will include establishing sites upstream and downstream of the lake on Opossum Creek and collecting data on water quality, temperature, and an initial evaluation of the benthic macroinvertebrates and stream fish communities. This research will translate to a long-term project to monitor fish and benthic macroinvertebrates in Opossum Creek and Lake Hydaway as the lake ages, progresses, and management decisions are made based on both recreational opportunities and biological communities. Current student(s) in Dr. Hedrick’s lab are focusing on the identifying and quantifying the mussel community in Opossum Creek.
The Hobson Lab – Synthetic Organic and Polymer/Materials Chemistry
Dr. Stephen Hobson
View Dr. Hobson’s biography
1. Molecularly imprinted polymer based sensor for per- and polyfluoroalkyl substances (PFAS)
Perfluorooctanoic acid (PFOA) is the archtypical PFAS resulting from the production and use of fluoropolymers such as Gortex® and Teflon ®. Its structure precludes environmental degradation and can lead to bioaccumulation in animals and humans. Molecularly imprinted polymers (MIPs) have widespread use for applications in the biomedical, analytical, chemical and biological sciences and as active materials in chemical and biological sensors. This ongoing project focuses on the following: synthesis of novel fluorinated crosslinking monomers; free radical polymerization in the presence of PFOA to form the MIP; and determination of the sensitivity and selectivity of the materials towards PFOA and other PFAS.
2. BGF synthesis and process optimization using microwave accelerated synthesis.
Bisphenol A (BPA) is a monomer used in the production of epoxy resins and as a solvent/developing agent in thermal printing processes (i. e. receipts). BPA is problematic both from an environmental/green chemistry perspective (current synthesized from non-renewable sources) and a toxicological perspective (estrogenic compound). The synthesis of known monomers with structures mimicking BPA but with reduced estrogencity has been reported and involves extended heating (24-36 hours) in polar protic solvents under strongly acidic conditions. Work-up required a large quantity of organic solvents and resulted in intractable emulsions. Final purification via flash chromatography results in mixtures of ortho and meta, products (~95:5) in moderate yield (35-60%). Using a CEM Discover 2.0 Microwave reactor optimization of the the yield and purity is under investigation. Variables to be examined include but are not limited to solvent (MeOH, EtOH, n-PrOH), temperature, pressure, reaction time, workup/extraction conditions, and purification.
The Howell Lab – Shoes: The Biomechanics of Walking
Dr. Daniel Howell
View Dr. Howell’s biography
Dr. Howell is interested in how shoes affect the anatomy of the foot and the biomechanics of walking. It is well-established that shoes alter the structure of the foot (causing hallux valgus, bunions, hammer toe, and ingrown nails, for example) and that shoes affect the physiology of the foot (e.g., altering body weight distribution and disrupting the windlass mechanism). It is believed that shoes with elevated heels, arch supports, and toe springs are a major cause of fallen arches. Dr. Howell developed a novel method of measuring arch heights from footprints and used this method to demonstrate that extended barefoot walking can increase arch height in low-arch individuals. Currently, Dr. Howell is using electromyography (EMG) to investigate the impact of high heels on muscle activity in the leg during ambulation.
The Isaacs Lab – Characterization of Predicted Histone-Modifying Enzymes from Cryptococcus Neoformans
Dr. Gary Isaacs
View Dr. Isaacs’ biography
Dr. Isaacs has been leading his research group on a more recent line of inquiry centered around SAS3 and other predicted histone modifying enzymes in Cryptococcus neoformans. It is well-known that histone modifications are used by the cell to control chromatin structure and regulate gene expression pathways. Specifically, histone acetylation is broadly associated with increased gene activity with deacetylation working to counteract those effects. His current focus has been to examine potential new players in this molecular balance and how they might modulate disease states.
Recently our group has purified recombinant SAS3 and created a custom polyclonal antibody for studying the protein in vivo. Our current and future goals are:
- Determine if SAS3 complexes can function as a histone acetyltransferase in vitro
- Identify components of endogenous SAS3 complexes
- Determine genomic targets of SAS3 action.
Our methodology includes, His-tagged purification of recombinant proteins, antibody purification and validation, acetylation assays, silver staining / SDS-PAGE, and chromatin immunoprecipitation (ChIP).
The Kalu Lab – Pathophysiology of Alcoholic Liver Disease
Dr. Ben Kalu
View Dr. Kalu’s biography
According to the Center for Disease Control, chronic liver diseases and Alcoholic Liver Disease are the 12th leading cause of death in the U.S., accounting for about 34,000 deaths in 2011. Also, liver cancers are the 8th leading cause of cancer death in the U.S. with about 16,000 deaths per year. A common factor associated with these liver diseases is chronic alcohol consumption. Therefore, our group is interested in elucidating the molecular mechanisms and pathophysiology of alcoholic liver disease and its course/progression to liver cirrhosis and hepatocellular carcinoma.
We use in-vivo models of chronic alcohol consumption as well as alcohol-treated cell culture models to examine:
- Genes whose expression levels are altered and the physiological impacts of such alterations
- Subcellular localization, integrity and functions of organelles with particular emphasis on the mitochondria (oxidative stress) and lysosomes (autophagy)
- Possible molecules, drugs or nutritional supplements that may reverse, retard or halt the development of progression of these disease conditions (drug discovery)
Our group is very hands-on and student-oriented, affording the students flexibility to work at their individual paces while giving them exposure to laboratory skills and analytical competencies that will help define their career paths and make them competitive in the science community.
The Korn Lab – Organic Compounds, Chemical Education, and Materials
Dr. Michael Korn
View Dr. Korn’s biography
Dr. Korn’s research interests are at the interface between organic chemistry, materials science, and biology and include the following topics:
- New Fluorophores. We are interested in developing fluorophores based on four different cores. This project involves the synthesis and analysis of these compounds, along with determining suitable biomedical applications.
- Development of New Organic Chemistry Laboratory Experiments. In this project, we are seeking to develop new and to improve existing experiments for the undergraduate organic chemistry laboratory. Projects include column chromatography, bromination reactions and the use of enzymatic reactions suitable for the organic laboratory.
- Planarity in Organic Semiconductors. We are interested in how side-groups attached to a specific aromatic core will affect the compounds’ resulting geometry and bandgap. Employing molecular modeling will assist in computing electronic properties.
- Crystal Growth on Titanium Substrates. In collaboration with the engineering department, we are interested in how titanium-based substances will grow on various titanium surfaces.
The Mirabelli Lab – Neuropathic Pain
Dr. Ersilia Mirabelli
View Dr. Mirabelli’s biography
Neuropathic pain is a debilitating condition that significantly impacts the quality of life in affected individuals and remains the most challenging to treat since conventional therapies currently do not adequately resolve it. The scope of this research is to elucidate pathological circuits and mechanisms underlying neuropathic pain using mouse models for chronic pain in adult mice, and pure murine neuronal and glial cultures to determine the involvement of inflammatory effectors in-vitro. Ultimately, we aim to propose novel targets for therapeutic interventions.
The Moore Lab – Drug Discovery in the Context of Type-2 Diabetes Pathogenesis
Dr. William Moore
View Dr. Moore’s biography
Dr. Moore’s lab studies metabolism and glucose homeostasis at the cellular and molecular levels, with a particular emphasis on type-2 diabetes and its complications. His current research investigates the effects of small, naturally occurring compounds on adipocyte glucose metabolism as well as their potential probiotic or antibiotic effects on specific gut microbiome species linked to metabolic health or obesity. Additionally, Dr. Moore is exploring the potential of these compounds to disrupt glucose metabolism in renal cell carcinoma.
The Mossé Lab – DNA Analysis and Trace Evidence Explorations
Prof. Kristin Mossé
View Prof. Mossé’s biography
Current research projects include:
Ballistic Cartridge Casing Library Project: where students are developing a database through the analyzation and comparison of cartridge casings fired from unknown and known firearms.
Civil War Surgical Table Project: where students are Generating DNA profiles from the Hillsman House surgical table used during the last battle of the Civil War, as well as determining the best method of sample collection and analysis from a wooden substrate.
Conehead Mummy Teeth Project: where students are extracting DNA from Conehead mummy teeth to optimize a minimally destructive DNA extraction technique that still allows for the generation of full DNA profiles while leaving the tooth intact.
Dog/Cat Hair Microscopic Analysis Project: where students are conducting microscopic analyses of dog and cat hairs from various breeds, determining similar and different characteristics between dog breeds and doing the same for cat breeds while taking into account breed, gender, age (approximation), and diet (if known).
Fingerprint Fumigation Project: where students are developing a new methodology for fingerprint fumigation by using various dyes from markers and highlighters to reduce time constraints and simplify the process of increasing the visibility of latent fingerprints.
The Raner Lab – Biochemical Studies of Natural Products and Plant Peroxidases
Dr. Gregory Raner
View Dr. Raner’s biography
The first involves the evaluation of a variety of natural products (herbal medicines, essential oils, etc.) for potential health-promoting activities, or possible adverse effects when consumed with pharmaceutical drugs. Specifically, students learn how to culture human liver cells in the lab and evaluate specific biochemical markers associated with positive health effects, such as anti-oxidant action or chemo-preventive properties. High throughput enzymatic assays using High-Performance Liquid Chromatography (HPLC) or 96-well formatted fluorescence or luminescence kinetic assays are the primary tools associated with the project.
A second broad area of research is aimed at identifying novel peroxidase activities associated with a variety of different plant sources. The long-term objectives are to establish a library consisting of hundreds of crude plant samples and characterizing their peroxidase activities with regard to their abundance, stability and potential for use in biotechnological applications. Currently, the prototypical member of this family, horseradish peroxidase (HRP), is used for hundreds of applications, including large-scale biocatalysis, biomedical diagnostics (ELISA, western blotting etc..), biosensor technology, water treatment and a host of others. Limitations related to HRP in terms of stability, sensitivity to harsh conditions and non-selective activity suggest that a library of the type being developed would have great potential value in improving current applications and in the development of novel applications for this class of enzymes.
The P. Sattler Lab – Herpetology
Dr. Paul Sattler
View Dr. Sattler’s biography
Dr. Sattler is involved with both lab and field studies with amphibians and reptiles. He has been an active member of the Virginia Herpetological Society since 1987 and has held every elected office at one time or another. He currently serves as the co-editor of their journal, Catesbeiana. He participates in the VHS surveys and supervises the Field Notes section of the journal, updating and determining the distribution of amphibians and reptiles in Virginia.
Dr. Sattler is also using molecular genetic techniques, protein electrophoresis and DNA sequencing, to examine the extent of genetic differentiation in isolated populations of amphibians, particularly salamanders in the Appalachian Mountains of Virginia. The technique has been useful in uncovering cryptic species, those that cannot be determined using traditional morphological characters. Molecular techniques can uncover new species, and once the molecular markers are available, to determine the extent of that species’ range. Thus, field and lab studies are integrated to answer basic questions on the distribution of animals in Virginia. Students are involved with research projects to isolate DNA from tissues, usually tail clips, and then PCR used to amplify specific gene fragments to either differentiate or identify species.
The G. Sattler Lab – Migration Dynamics of Northern Saw-Whet Owls
Dr. Gene Sattler
View Dr. Sattler’s biography
Northern Saw-whet Owls are a rare breeder at high elevations in Virginia and a secretive migrant throughout its range. Prior to initiating a project banding them during migration, only two historical records existed for the Lynchburg area. However, with the help of biology students, we have banded over 500 saw-whets at our banding station since 2002.

The project is focused on exploring the migration dynamics of this species as we answer questions about the timing of the migration in this region, differences in the magnitude of flights among years, and differences in the timing and magnitude of movement among age and sex classes. A banding station here also adds insights into the geographic distribution of Northern Saw-whet Owls during migration; we are one of the more southern banding stations for the species in eastern North America, and our location in the inner Piedmont of Virginia complements saw-whet banding stations in Virginia in the Ridge and Valley province to the northwest and in the outer Piedmont and Coastal Plain provinces to the east. The project also provides students with valuable field experience in mist netting, handling, and banding birds.

A second project has been studying the fall hawk migration from Liberty Mountain since 1997. Over a dozen species of hawks and other diurnally migrating raptors such as eagles and osprey migrate through Virginia each
year. The study of their migration dynamics in Virginia has always been done predominantly along mountain ridges of the western mountain and valley region and along the coast of the eastern Coastal Plain. We are documenting this migration in the inner Piedmont region to fill in gaps in our understanding of Virginia’s hawk migration.
The Snyder Lab – Forensic Chemistry Research
Dr. Chad Snyder
View Dr.Snyder’s biography
Forensic Chemistry Research
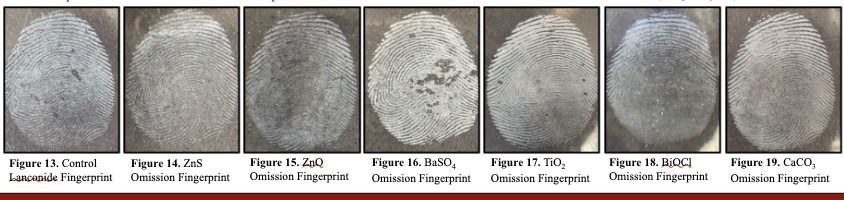
Inorganic Chemistry of Fingerprint Powder Formulation The Snyder group actively researches latent fingerprints and how to improve fingerprint powder mixtures. Approaching fingerprint powder formulation from the perspective of inorganic chemistry creates many opportunities to improve this relevant and highly valued method still used today for identification. The application of inorganic chemistry within the context of Ksp, color, tactility, safety, and density provides improved results for adhering to human sweat, oil, or other natural secretions on the skin.
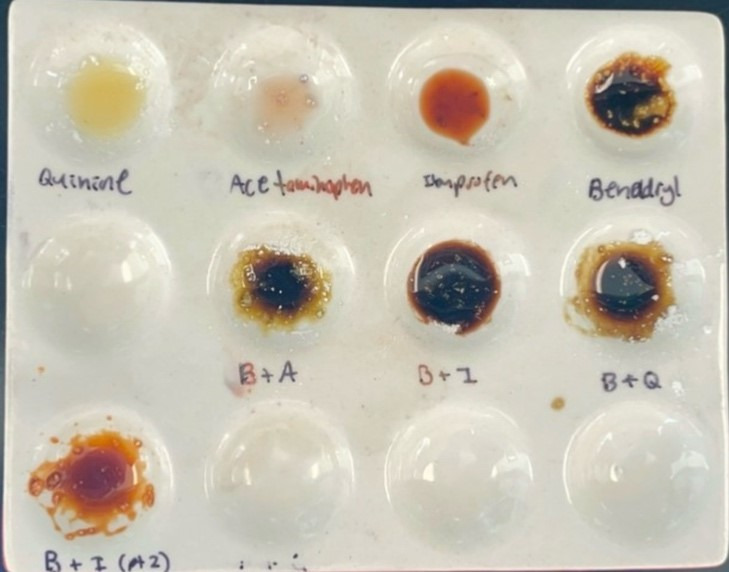
Organic Chemistry of Presumptive Color Tests The Snyder group also actively researches improvements in color tests for a variety of adulterants found within illegal drug samples using synthetic organic chemistry. The group only examines, formulates, and tests over-the counter organic compounds that have been proven to be cutting agents in illegal drugs as documented in peer-reviewed scientific publications. The students are trained in synthetic organic chemistry with the goal to make improved presumptive tests that identify these adulterants in the visible spectrum.
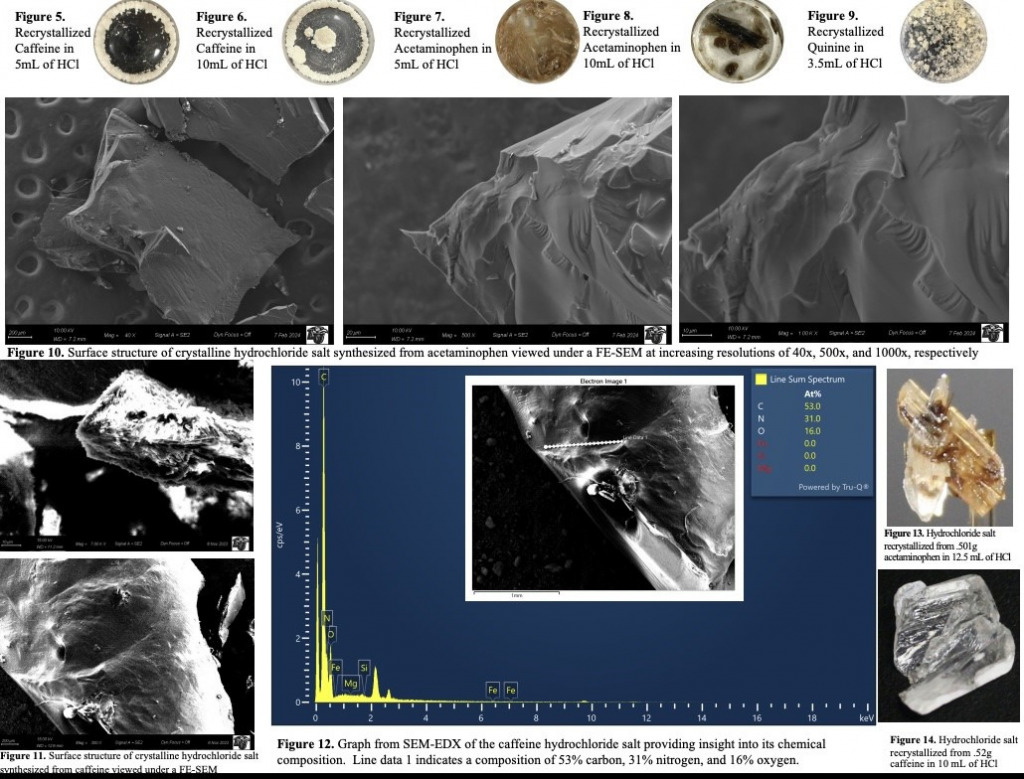
Scanning Electron Microscopy (SEM) of Over-the-Counter Hydrochloride Salts Used as Cutting Agents The student to participates in this research learns how to generate various hydrochloride salts of common over-the-counter medications often present in illegal samples. This synthesis is accomplished via organic chemistry and characterization is performed using MP, IR spectroscopy and SEM-EDX. The student is trained int eh organic synthesis as well as in IR spectroscopy. Additionally, the student learns to utilize for further analysis and characterization of these compounds as accomplished by students in this group (see Figure 3).
The Stevenson Lab – What effect does BPC have on the immune system?
Dr. Lindsey Stevenson
View Dr. Stevenson’s biography
Exposure to Bisphenol A (BPA) has been shown to have a range of deleterious effects, resulting in anything from life threatening cancers to cosmetic problems such as breast formation in men. However, BPA and other estrogenic compounds have been shown to affect the development and function of the immune system. The wide ranging harmful effects of BPA exposure have resulted in plastic manufacturers discontinuing the use of BPA in favor of “safer” BPA alternatives such as BPC, BPF, etc. But how safe are these alternatives?
Dr. Stevenson’s lab seeks to test the effects of exposure to BPA alternatives, with particular focus on how BPA and BPC affect numbers of B1 B cells that develop in the fish. High numbers of B1 B cells, unlike their classic B2 counterparts, have been correlated with predisposure to autoimmune diseases, including Lupus. At the moment, the Stevenson lab is creating a transgenic model of zebrafish, such that all B cells are tagged with green fluorescent protein (GFP), to aid us in our focus on how exposure to BPA and its alternatives alters development of B cells.
The Winter Lab – Insulin Resistance and mTORC1 Signaling
Dr. Jeremiah Winter
View Dr. Winter’s biography
Dr. Winter has been involved with the following:
- Studying insulin resistance as it pertains to mTORC1 signaling
- Elucidating the role of polyphenols in the regulation of mTORC1
- Treating Rat2 fibroblasts with large amounts of insulin to lower insulin sensitivity and make a cell culture model of insulin resistance
- Intracellular signaling of p300 and S6K1 as it relates to IRS-1 activity
- Understanding how the polyphenol Theaflavin 1 enters L6 cells
Dr. Winter has worked with undergraduate students and Ph.D. students, and his motivation is to see students take projects and make them their own. To accomplish this, the student must first be trained well, and Dr. Winter ensures that his students have a solid grasp of cell culture techniques that they can then take with them wherever they go.
The Xu Lab – Tissue Slicer – An Essential Tool for Ovarian Tissue Cryopreservation
Dr. Jing Xu
View Dr. Xu’s biography
Primary ovarian insufficiency (POI) can result from genetic disorders, autoimmune diseases, chemotherapy or radiation therapy, and exposure to hazardous substances. Ovarian dysfunction leads to delayed puberty in girls, as well as early menopause and infertility in women. Therefore, ovarian tissue cryopreservation is recommended to female patients at risk of POI, i.e., “freezing” ovarian tissue. “Thawed” ovarian tissue can be transplanted back to patients after medical treatment and recovery from illness with a goal of resuming ovarian function. This approach requires ovarian tissue slices with 1 mm thickness to be generated in a timely manner. Dr. Xu has a collaborative project with the School of Engineering to build a tissue slicer for ovarian tissue cryopreservation. The device will be tested for (a) the capacity of tolerating sterilization processes, (b) the biocompatibilty when processing fresh tissue without causing cytotoxic damages, and (c) the efficiency of generating ovarian tissue slices with desired thickness that maintain their integrity and viability after cryopreservation.